Latent and sensible cooling and heating equations - in imperial units
Sensible Heat
The sensible heat in a heating or cooling process of air (heating or cooling capacity) can be expressed as
hs = 1.08 q dt (1)wherehs = sensible heat (Btu/hr)q = air volume flow (cfm, cubic feet per minute)dt = temperature difference (oF)
Latent Heat
The latent heat due to moisture in the air can be expressed as:
hl = 0.68 q dwgr (2)orhl = 4,840 q dwlb (3)wherehl= latent heat (Btu/hr)q = air volume flow (cfm, cubic feet per minute)dwgr = humidity ratio difference (grains water/lb dry air)dwlb = humidity ratio difference (lb water/lb dry air)
- 1 grain = 0.000143 lb = 0.0648 g
Total Heat - Latent and Sensible Heat
Total heat due to both temperature and moisture can be expressed as:
ht = 4.5 q dh (4)whereht= total heat (Btu/hr)q = air volume flow (cfm, cubic feet per minute)dh = enthalpy difference (btu/lb dry air)
Total heat can also be expressed as:
ht = hs + hl= 1.08 q dt + 0.68 q dwgr (5)
Example - Heating Air
An air flow of one cfm is heated from 32 to 52oF. Using (1) the sensible heat added to the air can be expressed as:
hs = 1.08 (1 cfm) ((52 oF) - (32 oF))= 21.6 (Btu/hr)
Sensible Heat Load and Required Air Volume Chart
Sensible heat load and required air volume to keep temperature constant at various temperature differences between entering air and room air are indicated in the chart below:
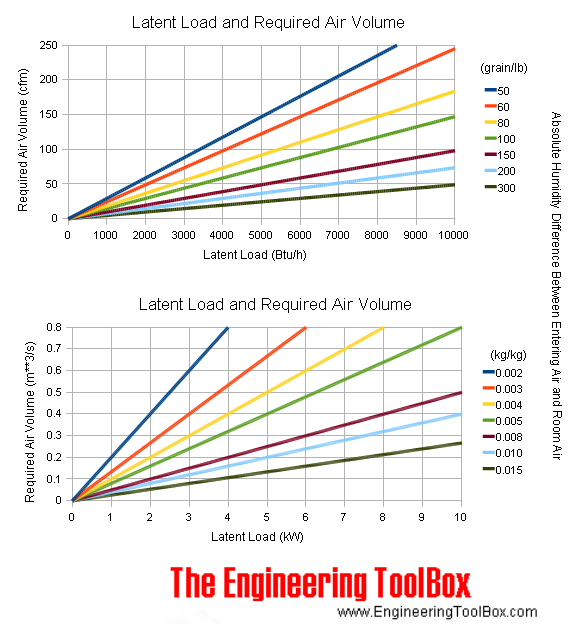
Latent Heat Load and Required Air Volume Chart
Latent heat load - humidifying and dehumidifying - and required air volume to keep temperature constant at various temperature differences between entering air and room air are indicated in the chart below:
SHR - Sensible Heat Ratio
The Sensible Heat Ratio can be expressed as
SHR = hs / ht (6)
where
SHR = Sensible Heat Ratio

If more people that write articles really concerned themselves with writing great content like you, more readers would be interested in their writings. Thank you for caring about your content. http://www.csacservices.com/
ReplyDelete